Contents
Introduction
As the common demonisation of cannabis in all its forms and derivatives finally unravels – thanks in no small part to the breakthrough of CBD into major markets – companies and researchers are at last beginning to look seriously at the possibilities offered by other cannabinoids.
All medications are a trade-off between benefits and side-effects. While cannabis has tremendous potential to address numerous health conditions, its psychoactive nature limits its usefulness as a medicinal product. However, cannabis is not simply one drug, but a combination of several active ingredients.
With extraction methods allowing cannabis’s composite parts to be better isolated (or, as is often the case, synthesised), it is now becoming possible to pick and choose specific therapeutic applications in exchange for fewer unintended side-effects.
Doing so, however, requires a more robust understanding of how specific cannabinoids operate. Here, then, is a brief review of some of cannabis’s lesser-known compounds – the so-called “minor cannabinoids” – with an emphasis on their potential applications.
From biosynthesis to the body
Let’s begin by quickly tracing the origins of some select minor cannabinoids that we will cover. 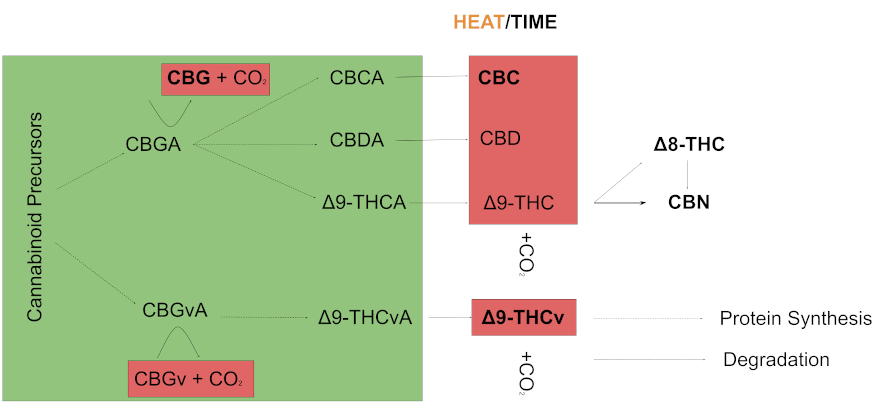
Arising from other precursors, cannabigerolic acid (CBGA) – the so-called “mother cannabinoid” – is metabolised into cannabichromenic acid (CBCA), cannabidiolic acid (CBDA), and delta-9 tetrahydrocannabinolic acid (delta-9 THCA). These are inactive cannabinoids which then degrade – or decarboxylate – into various cannabinoids (CBC, CBD, delta-8 THC, CBN) often with the aid of heat. Delta-9 tetrahydrocannabivarin (THCv) arises in a similar process through a different precursor.
Minor cannabinoids are thus often the downstream result of a compound degrading and gaining stability. The hundreds of cannabinoids that have been identified are not necessarily a product of cannabis evolution, but rather a reflection of chemistry. This raises a few considerations for how we should understand these compounds.
Minor cannabinoids are often found in limited concentrations, their composition largely the result of conditions unique to any given plant’s environment. For a long time – and still today to a lesser extent – extraction and isolation was difficult and unattractive for researchers (not to mention the legal restrictions that existed).
As extraction and cultivation methods are improved, alongside novel synthetic approaches to produce cannabinoids in the lab, research in this area is quickly developing. But as we will see the evidence base for any given cannabinoid is limited, with much of the support coming from animal models or cruder in-vitro studies.
Biological response
Thus, while it is important to review the science for each cannabinoid, it is just as important to understand the mechanism involved for any effect.
Although a given cannabinoid may not have been investigated for a certain condition, it may function in a similar way to one that has and may later be shown to have such treatment potential. Furthermore, a stronger case can be made for any particular mechanism of action, when multiple cannabinoids are shown to share properties. Indeed, this approach will be key to understanding future research developments.
Before diving into some of the literature, it is beneficial to quickly frame how cannabinoids produce a biological response.
Much of cannabis’s effect involves the body’s endocannabinoid system, a system of our own cannabinoids and receptors (CB1 and CB2 receptors) that play a key role in such areas as cognition, coordination, neurotransmitter activity, pain perception, and gastrointestinal function. Research is just beginning to document all the intricacies of this system.
CB1 and CB2 receptors are largely found in different areas of the body, which is key to understanding their function.
Receptors
CB1 receptors are found in the brain and spinal cord (i.e. the central nervous system) and play a key role in the psychoactive, behavioural and cognitive properties of cannabinoids.
CB2 receptors are largely expressed outside the central nervous system, being responsible for effects on the immune, gastrointestinal, and other systems. (More research is suggesting, however, that they play a larger role in the brain than once thought.)
Cannabinoids derived from the cannabis plant – so-called phytocannabinoids – can interact with these receptors by activating the receptor (agonism), blocking the action of the receptor (antagonism), or by interacting with our own cannabinoids. For example, they might block the re-uptake of an endocannabinoid such as anandamide (AEA).
Finally, cannabinoids can exert an effect on other bodily systems beyond the “canonical” endocannabinoid receptors CB1 and CB2.
Transient receptor potential (TRP) channels, for example, play a role in the body’s response to pain as well as some cancers. Yet as evidence mounts regarding these channels’ close association with cannabinoids, some scientists have called for them to be integrated into the endocannabinoid system. For now, this is largely just a question of nomenclature.
Let’s now turn our attention to five particularly significant minor cannabinoids: CBG, CBC, delta-8 THC, CBN and THCv.
Cannabigerol (CBG)
As the precursor for CBD, THC and CBC, cannabigerol develops early and is in very limited quantities in mature cannabis plants. It is non-psychoactive and displays a comparatively minor affinity for the CB1 and CB2 receptors. Its effect on CB2 has been shown to potentially aid in the treatment of inflammatory bowel disease, though the mechanism for that is not fully understood.
Outside the “canonical” endocannabinoid system, CBG is a potent TRPM8 antagonist (blocker), with evidence suggesting potential applications for cancer and pain treatments. It has also been shown to act as an alpha2-adrenoceptor agonist, which can potentially play a role in the treatment of pain. Further, CBG acts as a 5-HT1A receptor antagonist which may assist in the treatment of some nauseas.
One rather curious promise involves its application in the treatment of neurodegenerative diseases such as Huntington’s disease. While other cannabinoids are believed to be beneficial here through modulation of the CB2 receptor, CBG’s utility arises through other pathways, which are not yet fully understood.
Cannabichromene (CBC)
CBG eventually undergoes further processing by one of three enzymes, differentiating the compound into the acidic forms of either CBD, THC, or cannabichromene (CBC). Although to some extent the activity of these enzymes determines the relative amount of each cannabinoid produced, the largest factor is the particular genetics of a plant. So, while the acidic form CBC can be found in high concentrations in some plants, recent efforts in cultivation have significantly reduced its presence in order to maximise THC and CBD output. Therefore, unlike the other cannabinoids discussed here, CBC can perhaps be viewed as an “artificial” minor cannabinoid.
Similar to CBG, CBC displays a limited affinity for the CB1 and CB2 receptors and further exerts a very limited effect on the body’s natural cannabinoids. Accordingly, many of its effects arise from outside the endocannabinoid system.
CBC is a potent activator of the TRPA1 pathway, a potential target for pain management. It has promise as an anti-inflammatory agent, although the exact mechanism of this effect is unclear.
CBC also demonstrates potential as an innovative treatment for depression. As in the case of Huntington’s disease mentioned above, while other cannabinoids, such as delta-9 THC, have shown promise in this area, they generally exert an effect through the CB1 and CB2 receptors, whereas CBC’s effect appears to arise elsewhere.
Delta-8 tetrahydrocannabinol (Δ8 THC)
Chemically, delta-8 THC is best viewed as a product of delta-9 THC degradation, although in commercial use it is often synthesised from CBD.
Delta-9 THC’s infamous psychoactive effect arises from its activation of CB1 receptors. Agonism of CB1/ CB2 receptors is almost unique to the THC base structure; as a derivative of delta-9 THC, delta-8 THC is also psychoactive.
Limited evidence exists looking at possible effects of delta-8 THC, with most indications presenting a similar pharmacological profile to that of delta-9 THC. One advantage may be its reduced potency – that is, it functions as a sort of “diet THC”.
Yet the most significant consideration regarding delta-8 THC’s potential advantage over delta-9 THC is its relative stability. Delta-9 THC, and its acidic form, tend to degrade to cannabinol (CBN) – especially when exposed to heat – whereas delta-8 THC is much more stable, meaning a longer shelf-life for potential therapies.
Cannabinol (CBN)
CBN exists as a product of delta-9 THC degradation. Like delta-9 THC and delta-8 THC, CBN activates CB1 / CB2 receptors, yet its modest affinity for the CB1 means it is only mildly psychoactive. Because it serves as a sort of end product for delta-9 THC, it is found in very modest amounts in mature cannabis plants.
The most promising avenue for CBN appears to be its use as a topical agent. The compound has already completed two phase one clinical trials for treating epidermolysis bullosa, a rare blistering skin condition.
The company behind these trials also plans to investigate potential applications in the treatment of glaucoma, a common eye condition in which the optic nerve, which connects the eye to the brain, becomes damaged – usually caused by fluid building up in the front part of the eye, increasing pressure inside the eye.
Tetrahydrocannabivarin (THCv)
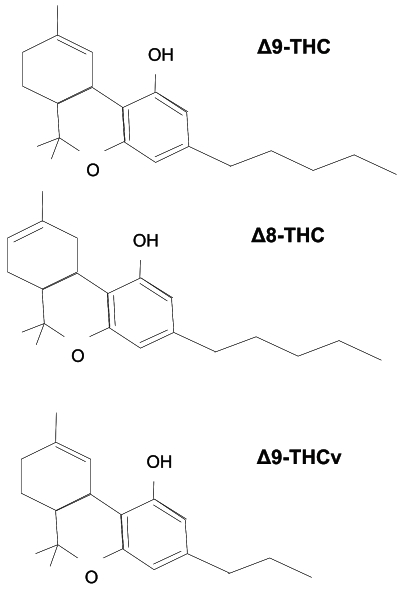 The biosynthesis of all cannabinoids is a complex process involving several precursors and intermediaries. Yet cannabinoids can be said to emerge after one such precursor, geranyl pyrophosphate, binds with either divarinolic acid or olivetolic acid – two types of carbolic acid differing only in their number of carbon atoms. Other than providing a basis for understanding the chemical structures of later cannabinoids, the genetic coding for these two compounds marks the beginning of genetic footholds to regulate cannabinoid content.
The biosynthesis of all cannabinoids is a complex process involving several precursors and intermediaries. Yet cannabinoids can be said to emerge after one such precursor, geranyl pyrophosphate, binds with either divarinolic acid or olivetolic acid – two types of carbolic acid differing only in their number of carbon atoms. Other than providing a basis for understanding the chemical structures of later cannabinoids, the genetic coding for these two compounds marks the beginning of genetic footholds to regulate cannabinoid content.
THCv is unique as the only cannabinoid here that derives from divarinolic rather than olivetolic acid.
The synthesis of THCv is almost exactly the same as delta-9 THC, with the end result lacking two carbons on its side chain (this results in what is known as a chemical homologue, a compound belonging to a series of compounds differing from each other by a repeating unit).
Looks, however, can be deceiving, and although delta-9 THC and THCv look similar they function differently.
For example, in contrast to the appetite stimulation properties of delta-9 THC, THCv is shown to promote a feeling of being full, suggesting it might be a possible treatment for obesity.
In the same vein, THCv shows promise as a potential treatment for type II diabetes, through mechanisms believed to operate outside the endocannabinoid system.
While a fair amount of research exists documenting therapeutic potential for THCv, little is known about its particular pharmacokinetics.
THCv has been shown in the lab to function as both a CB1 / CB2 agonist and antagonist depending on the dose at which it is administered. It displays more diverse action in living models, demonstrating a need for more specific research.
Better together? The entourage effect
While this report has so far focused on individual cannabinoids, another approach for researchers in the future is to embrace the fact that they are found together.
As cannabis gains greater legal acceptance, more data on its use will become available which both could and should be used to shed light on the effects of cannabinoids. However, better practices are needed in order to clearly document the relative amounts of each cannabinoid present, as well as clear methods of how to incorporate such findings into research.
A popular theme in this area is the idea of a polypharmacy, or “entourage effect”, the whole of cannabis being greater than the sum of its parts.
The effect is assumed to arise from cannabinoids’ interactions with and alongside one another – as well as with other, non-cannabinoid, compounds – which may enhance any given effect. While there is merit to this view, some are sceptical, particularly in the context of generating the evidence required to support a potential therapy.
In contrast to the development of a typical pharmaceutical, where a promising compound found in a laboratory progresses through increasing layers of scrutiny, generating an ever-stronger evidence base for a particular mechanism of action, the promise of cannabis is reversed, arising through its long, popular, and successful use as a compound with several active components (and potential mechanisms of action) all present at once.
If the evidence from widespread use of cannabis/cannabinoids is embraced, then combination or entourage effect arguments will prevail as it will be hard to attribute particular health effects to a single cannabinoid. If not, we may indeed want to start focusing on individual cannabinoid developments, more akin to the traditional monitoring of the pharmaceutical industry.
While much has been uncovered about the parts that make up cannabis, many questions remain about the effects and actions of minor cannabinoids.
Understanding any one of these is best achieved within the context of other similar cannabinoids, looking at potential shared mechanisms as well as the nature of the evidence and the evolution of the field as a whole.
All in all, minor cannabinoids possess immense promise as a means to leverage the medicinal value of cannabis by better targeting specific conditions. But for next steps – in the context of potential biomedical applications – it may be better to think about how regulators, the scientific community and others are picking up and understanding overall evidence rather than what could potentially be the next big health effect suggested by one study for a given minor cannabinoid (or combination of a few).
Potential applications of select minor cannabinoids
and mechanisms / site of action
| CBG | CBC | CBN | THCv | Δ8-THC | |
| Pain management | α2 agonist | TRPA1 agonist | Inconclusive | CB1 and CB2 | CB1 |
| Tumour suppression / cancer | TRPM8 antagonism | TRPA1 agonist | TRPA1 agonist | Unclear | |
| Neuroprotective | Reduction in mutant Huntington’s | CB1 agonism | CB2 agonism and CB1 antagonism | ||
| Inflammatory bowel disease | Reduction in NO production | TRPA1 agonist | TRPA1 agonist | ||
| Inflammation | Unclear CB2 and/or TRPA1 | Unclear | (Tested with synthetic analogue) | ||
| Bone health | CB2 antagonism | ||||
| Weight loss | CB1 antagonism | ||||
| Diabetes | Lipid liver accumulation inhibition | ||||
| Epilepsy | CB1 antagonism | CB1 | |||
| Schizophrenia | 5‐HT1A receptor activation | ||||
| Nausea | 5-HT1A antagonist | Unclear | |||
| Depression | Unclear | ||||
| Appetite stimulation | CB1 agonist | CB1 agonist | |||
| Opioid withdrawal | Unclear | Unclear | |||
| Glaucoma | Unclear | CB1 |
– Clayton Hale CBD-Intel contributing writer
Image: Yekaterina Kadyshevskaya, The Stevens Laboratory, University of Southern California






