 You can find CBD-Intel’s latest news on the European Commission here.
You can find CBD-Intel’s latest news on the European Commission here.
CBD’s entry in the European Commission’s database of cosmetics ingredients has been expanded to differentiate it from generic cannabis and by-products.
The move offers more clarity to the CBD cosmetic industry and is likely to aid its acceptance by regulators in EU member states.
In addition, the Commission’s Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs (DG GROW), now distinguishes between two types of CBD.
The new entries for cannabidiol distinguish between cannabidiol “derived from extract or tincture or resin of cannabis” and that which is “synthetically produced”.
The first entry now reads:
“Cannabidiol (CBD) as such, irrespective of its source, is not listed in the Schedules of the 1961 Single Convention on Narcotic Drugs. However, it shall be prohibited from use in cosmetic products (II/306), if it is prepared as an extract or tincture or resin of Cannabis in accordance with the Single Convention. Please note that national legislations on controlled substances may also apply.”
The entry for synthetically produced CBD contains the same text.
In order to clarify the distinction, CBD-Intel contacted the European Commission, which told us: “Cannabidiol is not included as such in the Schedules of the Single Convention on Narcotic Drugs of 1961. Therefore, we consider Cannabidiol outside the scope of entry 306 of Annex II to the Cosmetics Regulation 1223/2009. However, if it is prepared from banned substances such as extracts or tinctures or resin of Cannabis Cannabidiol would fall under the scope of the Convention and the prohibition II/306 should apply (hence the two entries in CosIng).” [Emphasis added by DG GROW]
So, though CBD is not itself a scheduled substance, problems may arise if it is prepared from substances which are, such as cannabis extracts.
In our view, problems with hemp-derived CBD will arise when it qualifies as an extract in the sense of the Convention. That would occur in cases where it has been extracted from the flowering or fruiting tops.
It is important to highlight, however, that the EU does not interpret or enforce laws, but leaves that responsibility to member states and their courts.
UN intervention
Commission officials, however, told CBD-Intel of possible change that might in the near future completely free CBD from suspicion and provide a clearer framework.
And that could follow the re-evaluation of CBD at United Nations level.
In January, UN secretary-general António Guterres wrote to the World Health Organization Expert Committee on Drug Dependence (WHO ECDD), recommending the UN Convention on Narcotic Drugs was amended so that:
“…preparations considered to be pure cannabidiol (CBD) should not be scheduled within the International Drug Control Conventions by adding a footnote to the entry for cannabis and cannabis resin in Schedule I of the Single Convention on Narcotic Drugs (1961) to read ‘Preparations containing predominantly cannabidiol and not more than 0.2 percent of delta-9-tetrahydrocannabidiol (THC) are not under international control’.”
Though the Commission says this letter has no legal standing, officials told CBD-Intel: “When these recommendations are voted and adopted at UN level, resulting in the amendment of Schedule I, we might have a clearer legal basis for potential changes regarding cannabidiol.”
The previous entry for cannabidiol in the Commission’s Cosmetic Ingredient (CosIng) database said simply:
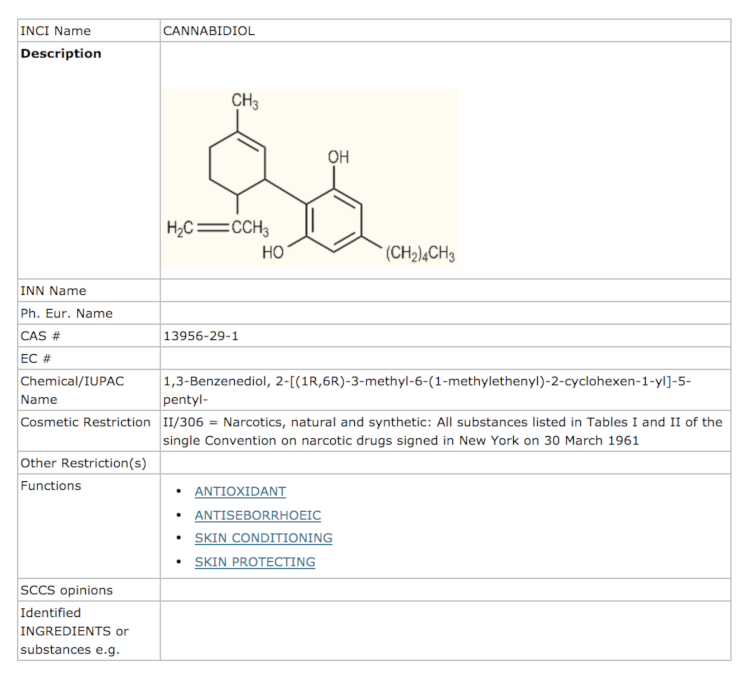
The introduction of extra details for CBD followed the modification a few months ago of the information in the CosIng database on cannabis extract and oil.
The new entry for cannabis could have been taken to imply that any type of extract, including CBD, would be considered illegal as there was no indication that the status of cannabis as a narcotic would depend on the level of THC.
After lodging a freedom of information (FOI) request with the EU, from which CBD-Intel obtained internal documents and communications, we inferred that at that time, the EU interpretation was that cannabidiol and other extracts of cannabis could be deemed illegal as an ingredient in cosmetics – even though our research had found a different interpretation among individual member states.
Questions in Parliament
The CosIng database is the European Commission’s database for information on cosmetic substances and ingredients contained in Cosmetics Regulation (EC) No 1223/2009. Inclusion does not mean a product can legally be used in cosmetics products – the database is primarily an “inventory of cosmetic substances and ingredients, employed for labelling cosmetic products” throughout the EU.
But while it is not binding – and member states have the last word when implementing and interpreting EU cosmetic rules – it is likely to be reflected in the interpretation of the applicable law by most regulators in EU member states.
Anecdotal evidence suggests that authorities in some countries rely on the database to form their judgements on whether an ingredient should be permitted. For example, CBD-Intel saw evidence of Slovenia openly questioning CosIng-related EU institutions about their interpretation, in order to form its own interpretation.
Meanwhile, Marco Zullo, who represents Italy’s Five Star Movement in the European Parliament, has put an official question to the Commission, asking if it would soon adopt legislation to harmonise the Europe-wide market for food and cosmetic products containing cannabis extracts.
He also questioned the reasons for the Commission’s recent decision “to exclude cannabis derivatives such as cannabidiol and hempseed oil from the register of permitted cosmetic ingredients”.
What This Means: Previously, the European Commission’s position was that “provided cannabidiol qualifies as an extract of cannabis within the meaning of the [Single Convention of Narcotic Drugs], it should then be prohibited from use in cosmetics”.
Recent changes can be seen as an attempt to bring clarity, implying that EU institutions may not consider pure CBD “regardless of its source” as a problematic ingredient for cosmetics. However, it is still not completely clear in what circumstances CBD will be considered “prepared as an extract or tincture or resin of cannabis” and therefore illegal.
In our view, the new entry has not changed the situation dramatically, but it clearly acknowledges that there are circumstances in which CBD will be allowed in the market. There will be other scenarios in which it is not – and our interpretation is that this would arise when CBD has been extracted from the flowering or fruiting tops of Cannabis sativa in the terms of the Single Convention on Narcotic Drugs.
While there is still considerable room for interpretation – and the decision of the WHO will be highly influential – the changes to the CosIng database at least show that the EU is thinking about CBD.
Since this article was written, the European Commission (EC) has informed CBD-Intel of its preliminary conclusion that CBD extracted from the whole Cannabis sativa plant will be allowed in cosmetic products in the EU.
The EC said that, although the recent decision by the Courts of Justice of the European Union in the Kanavape case did not specifically reference the regulatory framework governing cosmetics, its conclusions could still be applied.
– Pablo Cano Trilla CBD-Intel staff
Photo: Monfocus







